Essure
The Essure Birth Control device has been very controversial, because Bayer has been hiding behind their pre-market approval or preemption for many years. We have been speaking with women who have experienced unimaginable suffering often times resulting in a hysterectomy as young as 25 years old since 2013.
In late 2015, we were able to solidify a team of powerful law firms to pursue and fight against all odds for these strong women. Now we have momentum, and we are making great strides. We have high hopes for this mass tort as these women deserve their day in court.
Manufacturer
Bayer
Adverse Events
- Severe Cramping
- Taste of Metal
- Severe Bleeding
- Severe Pain
- Punctured Organs
- Nickel Poisoning
- Hysterectomy
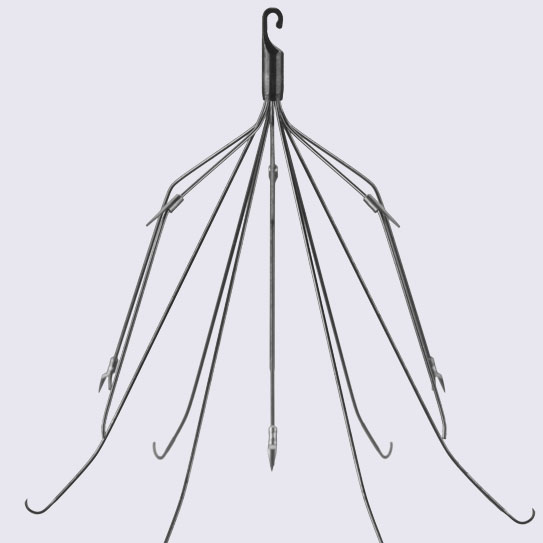
IVC Filter
An inferior vena cava (IVC) filter is a medical device implanted in the body’s largest vein to help block blood clots; however many IVC filters can result in hemorrhaging, pulmonary embolism, stroke, and death due to breakage and migration of the filter.
Currently, there are several lawsuits against different filter manufacturers on behalf of patients who have suffered serious injuries and even death as a result of these defective blood clot filters.
Manufacturer
C.R. Bard
Cook Medical
Cortis
Adverse Events
- IVC Migration
- IVC Fracture
- IVC Tilt
- IVC Irretrievable
- Organ Perforation
- Hospitalization
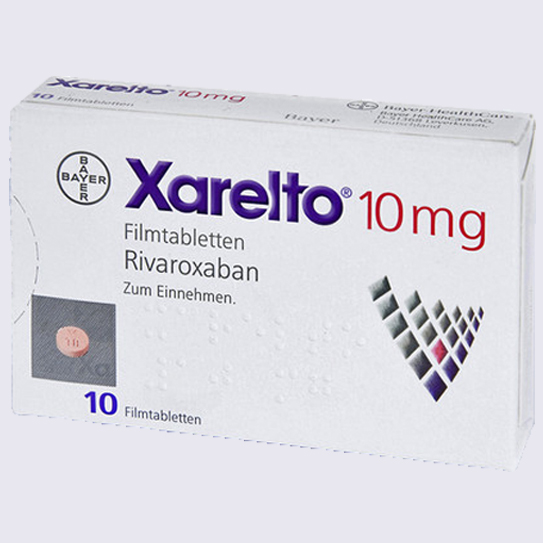
Xarelto
Xarelto is a blood thinner used to treat blood clots in patients. All anticoagulants prevent the formation of dangerous blood clots, however a major difference with Xarelto and traditional blood thinners like warafin is the existence of a reversal agent.
There is no antidote available to stop bleeding in patients taking Xarelto, and many patients have been hospitalized due to uncontrollable bleeding often times requiring blood transfusions to save the patient’s life, however, many people have bleed to death as a result of Xarelto.
Manufacturer
Bayer Healthcare AG
Marketed by
Johnson and Johnson Janssen Pharmaceuticals in the United States
Adverse Events
- Internal Bleeding
- Gastrointestinal Bleeding
- Intracranial Bleeding
- Stroke
- Blood Transfusions
- Hospitalization

Risperdal
Risperdal is indicated for the treatment of schizophrenia, acute manic episodes associated with Bipolar disorder, and irritability associated with autistic disorder. However Risperdal was used off label to treat obsessive compulsive disorder (OCD), attention deficit disorder (ADHD), Tourette’s syndrome, and Asperger’s disorder.
Risperdal was linked to Gynecomastia (breast tissue growth and swollen nipples) in adolescent boys. Gynecomastia is the overdevelopment of male breasts usually caused by too much estrogen or too little testosterone. Many boys were forced to undergo liposuction or even a mastectomy to remove the breast gland tissue.
Manufacturer
Johnson and Johnson Janssen Pharmaceuticals
Adverse Events
- Gynocomastia
- Swollen breast tissue
- Nipple discharge
- Female breast appearance in young boys often times resulting in a mastectomy
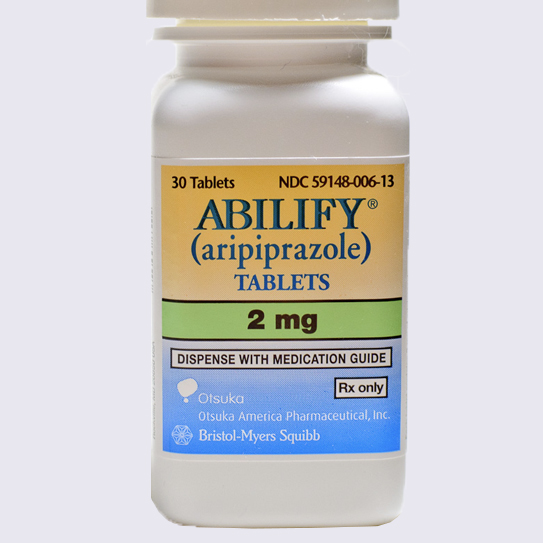
Abilify
Abilify is one of the most commercially successful pharmaceutical drugs of all time. The blockbuster drug has been marketed as a complementary treatment to anti-depressants, catapulting it to the top of the pharmaceuticals best-sellers list.
Abilify (aripiprazole) is a second generation antipsychotic used to treat certain mental health disorders and their symptoms, including schizophrenia, manic and mixed episodes associated with Bipolar I Disorder, Major Depressive Disorder (MDD) in combination with other drugs, irritability associated with Autistic Disorder, and Tourette’s disorder.
Abilify has been the subject of numerous FDA warnings since its approval in 2002, including most recently in May 2016, when the federal agency warned the drug could cause compulsive or uncontrollable urges to gamble, binge eat, shop and have sex in certain patients. Some people taking Abilify have reported accruing tens of thousands of dollars in debt because of these drug-related compulsions.
Manufacturer
Otsuka Pharmaceutical; marketed in the U.S. by Bristol-Myers Squibb
Adverse Events
- Compulsive gambling
- Compulsive sexual behavior; hypersexuality
- Compulsive eating
- Compulsive shopping
Taxotere
Hundreds of thousands of women are diagnosed with breast cancer every year in the United States. A majority of those women will undergo chemotherapy to treat their cancer, accepting a well-known and common side effect of this treatment: temporary hair loss. What many women did not know was that the temporary hair loss they were experiencing from their treatment was not so temporary after all.
The FDA has warned the most widely prescribed drug to treat breast cancer, Taxotere (docetaxel), can cause permanent hair loss in some patients. Many women speaking out against the drug say they would not have taken it had they known there was a risk of permanent hair loss.
Manufacturer
Sanofi-Aventis
Adverse Events
- Alopecia (permanent hair loss)
Cipro-Levaquim
Cipro is the brand-name of a popular prescription antibiotic medication used to treat common bacterial infections, including sinus infections, ear infections, bronchitis, pneumonia, and urinary tract infections. The main ingredient in Cipro, ciprofloxacin, belongs to a class of drugs called fluoroquinolones. Cipro and other fluoroquinolones are among the most-prescribed antibiotic medicines in the United States, with over 26 million people prescribed one of six currently available fluoroquinolones annually.
In May 2016, the FDA issued a warning about Cipro and other fluoroquinolones saying the drugs’ serious side effects do not usually outweigh the benefits for patients being treated for uncomplicated UTIs, sinus infections and bronchitis. The agency’s safety communication warned fluoroquinolones, including Cipro, are “associated with disabling and potentially permanent serious side effects that can occur together. These side effects involve the tendons, muscles, joints, nerves and central nervous system.”
Manufacturer
Bayer Healthcare
Adverse Events
- Aortic dissection
- Aortic aneurysm
- Peripheral neuropathy (nerve damage)
Bair Hugger
The Bair Hugger warming system is used in more than 80% of U.S. hospitals to keep patients warm as they undergo surgery. It is currently the number one choice in patient warming systems and consists of a warming unit and a blanket that is draped over the patient.
Bair Hugger’s forced-air system has been associated with an increased risk of deep joint infection in patients undergoing knee or hip replacement surgeries. Some studies suggest the forced-air system circulates contaminated particles from operating room floors over the surgical site, increasing the risk for infection. Patients who undergo hip and knee replacements are especially vulnerable to infections, and an infection in the prosthetic device could mean additional surgeries are necessary to clear the infection.
Manufacturer
3M
Adverse Events
- Surgical site infections, including MRSA
Invokana
Invokana (canagliflozin) was the first in a new class of drugs used to treat type II diabetes, a disease that afflicts nearly 30 million Americans. It is marketed as a simple, once-daily pill that can even help patients lose weight. Since its approval in 2013, Invokana has become the number-one prescribed drug in its class with more than 8 million prescriptions.
After only a few short months on the market, an increasing number of people with type II diabetes were being diagnosed with a serious condition called diabetic ketoacidosis after using Invokana. In May 2015, the FDA issued a safety communication warning of the risk of diabetic ketoacidosis in patients taking Invokana and other SGLT2 inhibitors. Diabetic ketoacidosis is a serious complication of diabetes and, if left untreated, can lead to kidney failure and death.
Manufacturer
Johnson & Johnson subsidiary Janssen Pharmaceuticals
Adverse Events
- Diabetic ketoacidosis
- Kidney failure
Viagra
The blockbuster erectile dysfunction drug Viagra has become one of the best-known brand names on earth. Viagra was the first-ever oral medication approved to treat male impotence when it came on the market in 1998.
The drug’s main ingredient, sildenafil, was originally tested as a treatment for high blood pressure and angina pectoris, a kind of chest pain caused by too little blood flow to the heart. Early clinical trials showed little success in the treatment of angina, but people participating in the trial reported an unexpected side effect: better erections.
Today, Viagra is still one of the best-selling ED medications on the market, but in spite of its popularity, there may be a dark side to the little blue pill. Recent studies have suggested the drug puts users at an increased risk for the deadliest form of skin cancer, melanoma.
Manufacturer
Pfizer Inc.
Adverse Events
- Melanoma
Zofran
Zofran was originally approved by the FDA in 1992 for cancer victims. In approximately 1996, GlaxoSmithKline found a new market for this drug, and marketed it “Off Label” to millions of pregnant women for morning sickness. Since then, there have been several medical studies linking Zofran to birth defects when used in the first trimester of pregnancy.
We have been helping thousands of women who seek justice for failing to warn them of the risks to their babies.
Manufacturer
GlaxoSmithKline
Adverse Events
- Atrial Septal Defect (infant)
- Ventricular Septal Defect (infant)
- Severe Heart Conditions (infant)
- Cleft Lip/Palate (infant)

Roundup
Farmers, landscapers, commercial nursery employees, forest industry workers, and many other people working with chronic Roundup exposure are mysteriously coming down with B-Cell and T-Cell lymphomas at an alarming rate.
Emerging science recently linked Glyophosate to Cancer. The World Health Organization did a full study in 2015 on Glyophosate, and they put it in the second highest category for probably causing cancer. Recently California’s Office of Environmental Health Hazard Assessment determined that Glyophosate was a substance known to the State of California to cause cancer.
Manufacturer
Monsanto
Adverse Events
B-Cell Lymphomas: Follicular lymphoma, chronic lymphocytic leukemia, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone B-Cell lymphomas.
T-Cell Lymphomas: Peripheral T-Cell lymphomas
MANY OF THE BEST AND THE BRIGHTEST LAW FIRMS TRUST US
Why Choose Us
We are a law firm first, and we are selective with who we work with. We are ethical, exclusive, and experienced and we never cherry pick cases for us or any law firm.
Ethical
Austin Majors has been in legal advertising for the past two decades and has always maintained the highest level of ethics.
Exclusive
We have a strict rule that we never participate in a campaign that is being sponsored. All of our campaigns are built exclusively for the sponsoring firm.
Experienced
Our marketing team has managed hundreds of millions of advertising dollars over the past 20 years, and we’ve had to return the investment on the quality of the cases as a law firm.